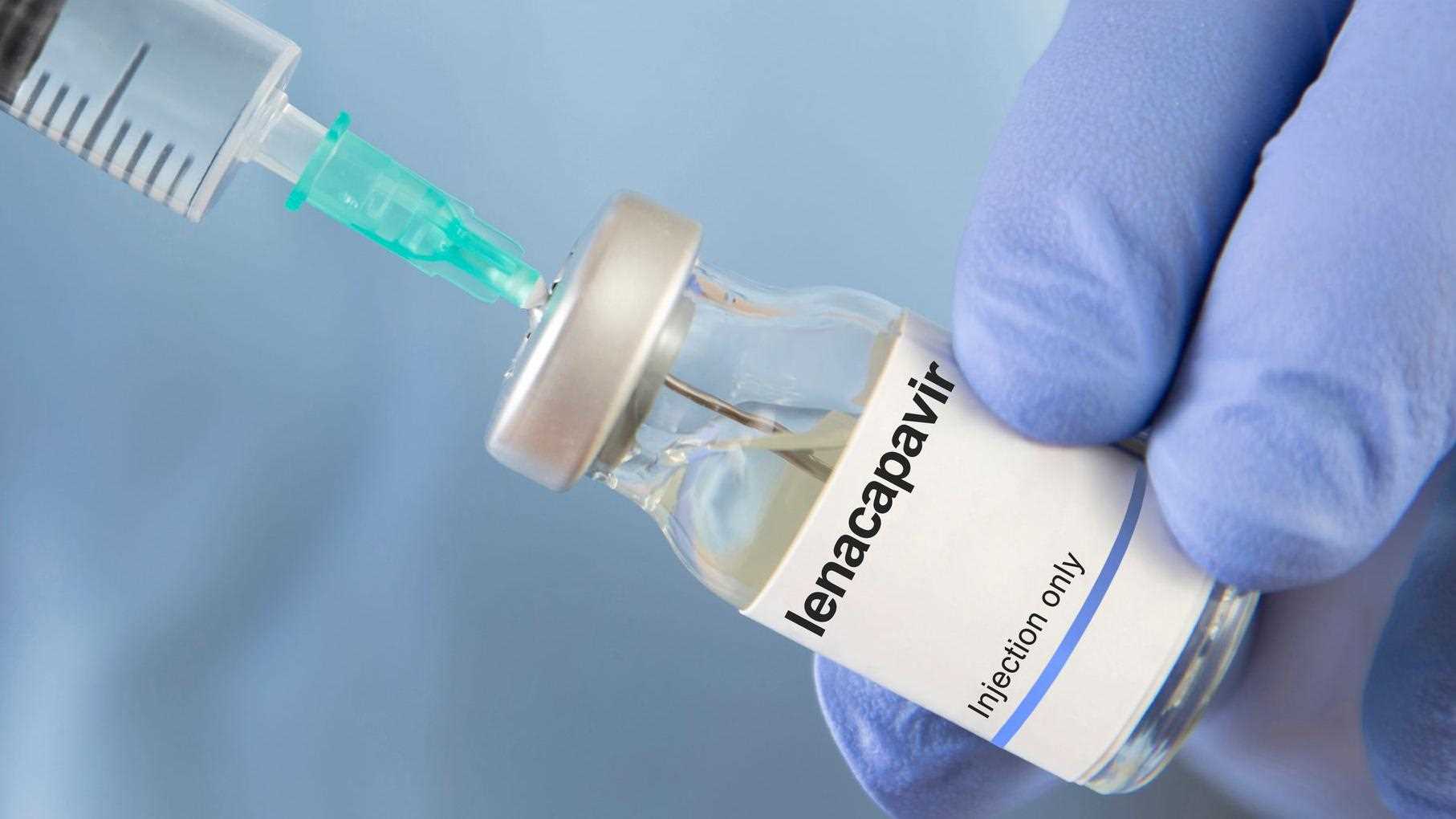
The Ministry of Health and Child Care has commenced the national rollout of Lenacapavir, a new long-acting HIV prevention medicine recently approved by the Medicines Control Authority of Zimbabwe.
Health Minister Douglas Mombeshora said the rollout is being supported by the World Health Organisation, local research institutions, the AIDS and TB Programme, PEPFAR, the Global Fund and other partners to ensure safety, proper monitoring and equitable access across communities.
Lenacapavir provides protection for up to six months from a single dose and is designed for people at substantial risk of HIV infection. Mombeshora said adolescent girls, young women, pregnant and breastfeeding women, key populations and mobile workers stand to benefit from the additional layer of protection offered by the new drug.
He stressed that Lenacapavir is meant to complement existing HIV prevention tools, not replace them.
“It does not replace the traditional ABC pillars of prevention — A for abstain, B for being faithful to one partner, and C for correct and consistent use of condoms,” he said.
Related Stories
Mombeshora cautioned that the drug must not be interpreted as approval for risky behaviour.
“Let me be clear that Lenacapavir must not be seen as a licence or visa to engage in risk behaviour. STIs and other communicable diseases remain prevalent,” he said, noting that the drug does not protect against those infections.
He added that responsible sexual behaviour, supported by the ABC principle, remains central to personal and public health.
The Minister said the Ministry will continue working with partners to ensure a controlled and well-monitored rollout of Lenacapavir within communities.
He said its introduction represents an important step in expanding HIV prevention options, while emphasising that behaviour change and informed choices remain at the heart of reducing new infections.


















Leave Comments